In a shocking turn of events, a woman in her mid-twenties from Newcastle upon Tyne found herself rushed to hospital after experiencing terrifying physical side effects following the use of a so-called cult beauty product. What began as a routine self-care ritual quickly escalated into a frightening medical emergency when she noticed her eyes beginning to swell and bulge alarmingly.
The young woman, described by friends as being extremely enthusiastic about affordable but popular skincare finds, had recently purchased a widely praised yet inexpensively priced beauty serum from an online retailer. The product had amassed a viral reputation, tweeted about by users for its impressive promotional before-and-after visuals and glowing reviews. Bargain seekers across social media platforms had flocked to acquire it, trusting its efficacy based on enthusiastic endorsements and glossy packaging.
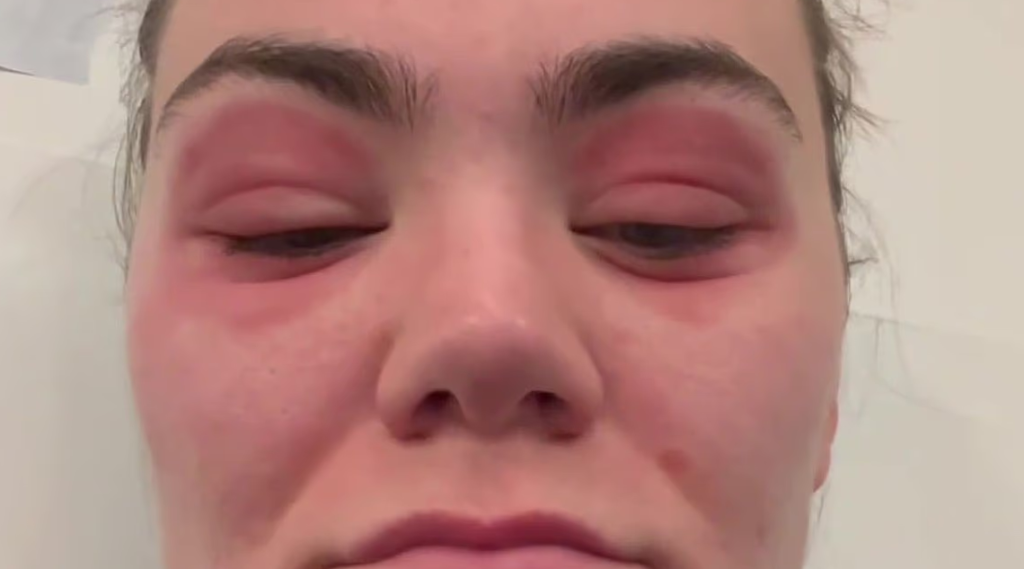
Shortly after applying the serum to her facial skin and around her eyes, however, the tone turned quickly alarming. Within just hours, she began noticing intense burning sensations in the delicate skin surrounding both eyes. It wasn’t just temporary discomfort—her eyes appeared to be literally bulging from their sockets, swelling to far beyond their typical size. Her eyelids ballooned and reddened, the texture turned taut and shiny, and she was suddenly facing an appearance so shocking that those around her demanded immediate medical attention.
Friends and family members acted fast, driving her to the nearest accident and emergency department. Upon arrival, hospital staff were taken aback by the severity of her presentation. Her eyes were visibly protruding, inflamed, and accompanied by severe pain and stinging. The medical team recognized this as a textbook example of a severe allergic reaction, likely linked to one or more corrosive or sensitizing agents in the serum—possibly undisclosed or improperly labeled chemicals.
Over the next two days of hospitalization, she underwent extensive treatment to reduce inflammation and manage the allergic response safely. Intravenous antihistamines, corticosteroids, and soothing cold compresses became her lifelines as doctors worked to reverse the damage. Eye scans and examinations ruled out internal damage to the optic nerves or orbital bone structure, offering some relief that the assault had not permanently damaged her sight. Still, the acute swelling raised alarm among medical staff, warning that had she delayed seeking help, she risked long-term vision impairment or even vision loss.
As doctors closely monitored her condition, the young woman struggled with intense fear over potential consequences. Though the swelling was trending downward, she was told to expect a prolonged recovery period; residual puffiness, sensitivity to light, and disrupted tear function were all possible lingering side effects. Additionally, she faced the emotional trauma of enduring such a distressing transformation of her appearance, which had upended her sense of normalcy and self-confidence.
Outpatient follow-up appointments were arranged, involving a multidisciplinary healthcare team: ophthalmologists to protect her vision, dermatologists to heal her skin, and allergists to identify specific trigger substances in the serum. Allergy patch testing was scheduled to pinpoint the offending ingredient. Meanwhile, her physician urged her to keep the serum container, packaging, and any receipts, to assist with ingredient analysis and potential consumer complaints or product recall.
On social media, the incident sparked a surge of reactions as reactions poured in from other users of the same beauty product. Some shared similar experiences of rashes, burning skin, or swollen eyes. Others expressed alarm at the notion that a low-cost, aggressively marketed skincare item could surge as a cult favorite without transparent testing or regulatory oversight. The situation has highlighted the vulnerability of online beauty trends that rely on hype rather than clinical evidence, as well as the dangers of eye-area products that may contain potent actives not intended for use so close to sensitive structures.
Consumer advocates have seized upon this case as a cautionary tale. They are calling for stricter labeling laws, tighter access to ingredient disclosures, and more rigorous regulation of beauty products sold online—especially those labeled with “natural,” “clinical,” or “ dermatologist-grade” claims that may lull buyers into a false sense of safety. Healthcare professionals, too, are reminding the public that the thin skin around the eyes carries heightened risk of adverse reactions and should be treated with extra caution when applying new cosmetics or skincare items.
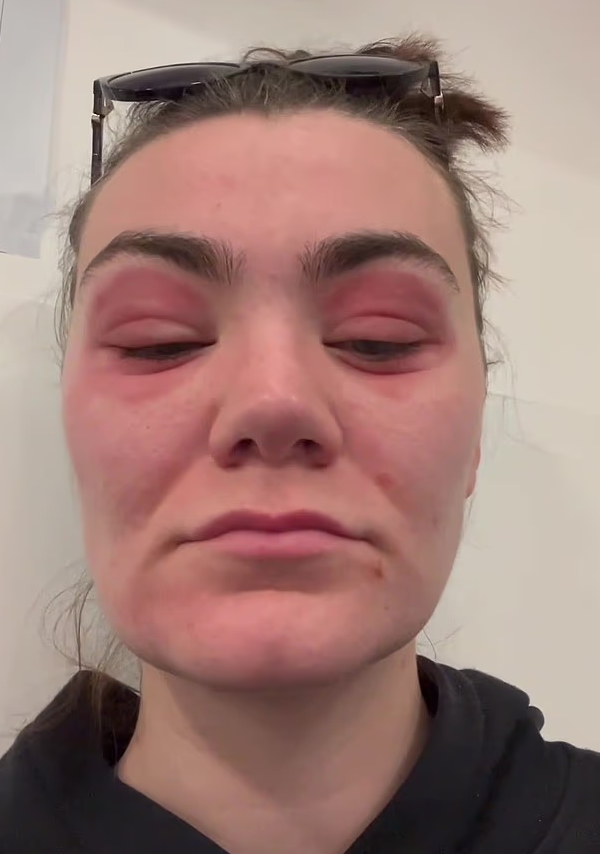
For now, the woman is recuperating slowly at home, still under careful professional care. She reports that her vision is slowly clearing and the swelling is receding, though she remains wary and reluctant to return to beauty products anytime soon. Her journey serves as a stark reminder that even seemingly harmless beauty buys can carry hidden risks—especially when battling for attention in the highly competitive online skincare market. Going forward, consumers are being urged to patch-test new products, seek out trusted sources, and monitor their bodies closely for signs of reaction.
As for the cult serum, its future now hangs in the balance. If further investigations reveal serious ingredient mislabeling or health hazards, the product could face recalls or bans. In the meantime, this Newcastle woman’s painful experience underscores a clear message: beauty trends are no substitute for safety, and every application carries responsibility—especially when it involves the eyes.

















