A previously healthy woman was hospitalized with severe liver damage after taking turmeric supplements a popular natural remedy. This herbal product nearly destroyed her liver. Find out more in the article below.
Supplements
The 49-year-old woman, who had no history of liver disease or alcohol abuse, began taking turmeric supplements daily. Within three months, she developed symptoms such as jaundice (yellowing of the skin and eyes), dark urine, fatigue, and nausea. Medical tests revealed that her liver enzymes were severely elevated, indicating acute hepatitis. After ruling out other causes—such as viral infections, autoimmune conditions, and alcohol or drug use—doctors determined that her liver injury was most likely caused by the turmeric supplement.
Turmeric: A Popular But Powerful Supplement
Turmeric, derived from the Curcuma longa plant, has gained popularity in recent years as a natural remedy for a wide range of conditions, including joint pain, inflammation, and digestive issues. The active ingredient, curcumin, is known for its antioxidant and anti-inflammatory properties. However, while turmeric is generally safe in food amounts, high concentrations found in supplements may pose risks for certain individuals. In this case, the supplement also contained black pepper extract (piperine), which is commonly added to enhance curcumin absorption—but may also increase its potency in potentially harmful ways.
Doctors Sound the Alarm
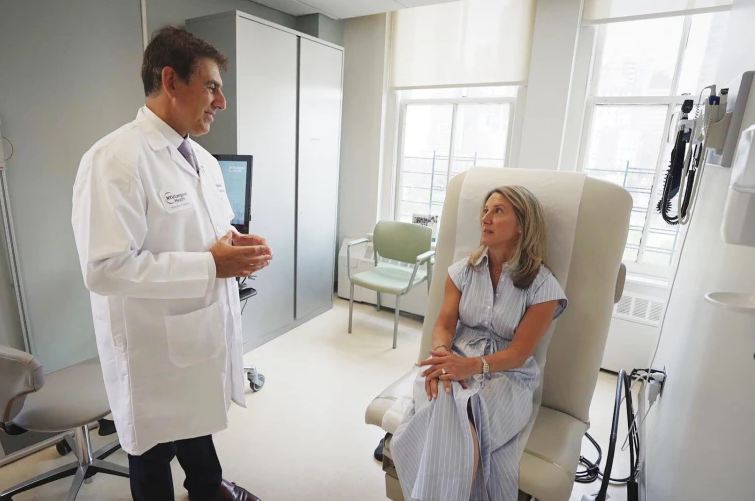
Physicians at the Henry Ford Health System in Michigan, where the woman was treated, have published the case to raise awareness. They emphasize that even natural products can have powerful effects on the body and should not be taken without medical supervision.
“Just because something is natural doesn’t mean it’s completely safe,” said Dr. Dylan Daniel, one of the treating physicians. “Supplements are not regulated in the same way as prescription drugs, and their interactions with the body—and with other medications—are often poorly understood.”
FDA and Supplement Oversight
In the United States, dietary supplements are not subjected to the same rigorous approval process as prescription medications. Manufacturers are not required to prove safety or efficacy before marketing their products, which has led to growing concerns among health experts. The Food and Drug Administration (FDA) does monitor supplements post-market and can take action against unsafe or misbranded products—but often only after adverse events have been reported. This case adds to a growing body of evidence suggesting the need for tighter regulation and public education on supplement safety.
The Woman’s Recovery and Cautionary Tale
Fortunately, after stopping the supplement and receiving supportive care, the woman made a full recovery. Her liver function gradually returned to normal, and she no longer experiences symptoms. However, her case serves as a stark reminder that even widely accepted natural products can cause harm if taken inappropriately or without guidance.

Conclusion: Use Supplements with Care
This incident underscores the importance of consulting a healthcare professional before starting any supplement, especially in high doses or over long periods. While turmeric may offer genuine health benefits, it also carries potential risks—especially when concentrated in pill form. As the popularity of natural remedies continues to grow, so does the need for responsible use and better public understanding of their effects.